关于IVD设备的电磁兼容以及安规测试,都一直是大家忽略的部分。的确,这种类型的设备在欧盟不管是IVDD还是IVDR,都是属于自我宣称居多,在FDA也是510(k)豁免。但是既然是有源的产品,本质上当然还是需要做电磁兼容和安规测试啦。
在EMC草稿指南中,就专门有提及这点:
Most in vitro diagnostic devices (IVDs) are outside the scope of 60601-1-2. In the absence of a recognized EMC standard for IVDs16 at the time of this guidance publication, we recommend using the test methods in IEC 61326:2-6, using acceptance criteria specific to the device’s functions and intended use, and using the test levels specified by 60601-1-2 for the device’s intended use environment.
EMC草稿指南沿用了FDA关于产品风险等级指南的分类“Factors to Consider Regarding Benefit-Risk in Medical Device Product Availability, Compliance, and Enforcement Decisions Guidance for Industry and FDA Staff”,将有源产品的风险分成3类:
Medical device-related deaths and serious injuries include events (including procedure-related complications) in the use of the medical device that have caused or could cause or contribute to a death or injury or illness that is life-threatening, results in permanent impairment or damage to the body, or requires medical or surgical intervention to prevent permanent harm to the body.
Medical device-related non-serious adverse events include events (including procedure-related complications) in the use of the medical device that have caused or could cause or contribute to minor, temporary or medically reversible injuries that do not meet the criteria for classification as a medical device-related serious injury.
Medical device-related events without reported or potential harm include medical device nonconformities that have no related harm, medical device malfunctions that have no related harm, and procedure-related complications with no related harm.
在过去的EMC测试中,是没有这个风险级别分类的。在EMC草稿指南中引用了这个概念,就使有源产品的EMC风险分类,基本上与软件验证风险分类、网络安全测试风险分类的要求是一致了,这使很多测试的归类上,更加统一了。并且在日后判定需要做什么测试,什么测试不需要做的基础上,更加有迹可循了。
在过去的指南,包括现行的60601系列标准,都主要是针对讨论电磁干扰对设备安全性方面的影响的。但在新的EMC草稿指南中指出,制造商除了要考虑安全性外,也应该考虑在电磁干扰的环境下,设备有效性的影响:
We recommend conforming to IEC TR 60601-4-2 Medical electrical equipment - Part 4-2: Guidance and interpretation - Electromagnetic immunity: performance of medical electrical equipment and medical electrical systems to assess the immunity of the performance associated with the intended use because the test methods are similar to 60601-1-2 and can be tested at the same time.
在过去的指南中,基本上就是两个使用环境的选择,要么就是医疗环境,要么就是非医疗环境,但在新草稿指南中,FDA对设备的使用环境,提供了多一个选择,并且也用了例子说明:
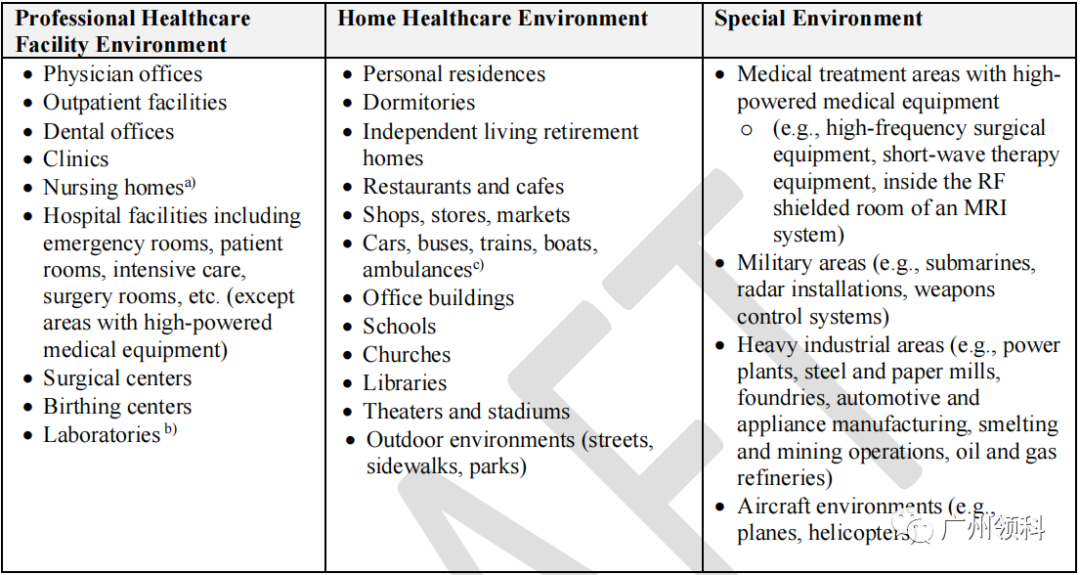
在Special Environment中,可以看到有很使用场景,是以前不敢想象的。可以看出FDA在撰写新指南的时候,也是充分考虑了当前科学技术的发展。
新草稿指南中指出,在进行EMC测试时,要结合产品使用场景,想象合理的电磁干扰来源以及可能被干扰的设备,进行符合实际应用情况的EMC测试。
随着科学技术的发展,近几年来各种无线网络、蓝牙操控的医疗器械或日常家用电器产品越来越多,FDA在撰写新指南的时候,也考虑上这个情况。
根据新指南要求,分类等级为上文1、2级别的设备,都需要进行无线干扰测试:
For medical devices in the risk category “Medical device-related deaths and serious injuries” or “Medical device-related non-serious adverse events” we recommend that:
testing be performed according to FDA-recognized consensus standards (e.g., FDA- recognized AIM 7351731 Medical Electrical Equipment and System Electromagnetic Immunity Test for Exposure to Radio Frequency Identification Readers - An AIM Standard for RFID emitters), or equivalent EMC test methods, with justification. If no consensus standards exist, specific immunity testing should be performed to demonstrate that the medical device is safe with regard to each identified emitter that is foreseeable in the intended use environment; and
labeling be specific to the risks to patients and operators and include any mitigations and warnings needed, based on the test results.
这个要求是新增的,在过往都没有,希望大家要注意了。

以上就是EMC新草稿指南的要点分享了。老实说,如果过去是认认真真做的话,其实本质上新指南的要求并没有增加,最多就是无线干扰测试的要求范围变广了。但是鉴于过去一些混乱的情况,估计对某些制造商来说,会是亚历山大吧。建议大家有空都看看完整版本,提前准备了。
参考来源:
[1] Electromagnetic Compatibility (EMC) of Medical Devices